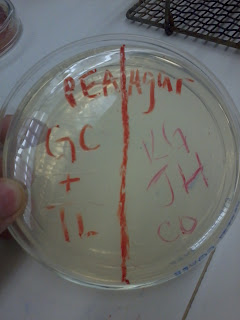
|
| Top: Antibiotics, Bottom: Oil |
Antibiotics
|
Oils
|
||
1. Penicillin
|
No inhibition
|
1. Clove
|
10 mm
|
2. Erythromycin
|
18 mm
|
2. Cinnamon
|
20 mm
|
3. Chloramphenicol
|
20 mm
|
3. Thyme
|
22 mm
|
4. Novobiocin
|
No inhibition
|
4. Eukalyptus
|
No inhibition
|
5. Augmentin
|
15mm
|
5. Ginger
|
No inhibition
|
The best that worked on our bacteria out of the tests was thyme, with Cinnamon and Chloramphenicol coming in second. Over all, the oils had a better inhibition of the bacteria than the antibiotics. Our gram negative bacteria showed similar results to others in the class who also had gram negative bacteria. Out of the oils, in general, the thyme, cinnamon, and gloves tended to work best on gram negative. For the antibiotics, the chloramphenicol worked the best. Again, this worked in general for gram negative bacteria because it bypassed other systems and attacked the protein synthesis.
We also checked our results from the Hamburger extract experiment. What was an indication of the antibody reaction was a small crescent shaped cloud in between where the hamburger extract was and the anti solutions surround it. The only antibody that was formed in our result was the anti-bovine. This means that cow meat was really the only meat used in this hamburger.
 |
| Crescent seen between top right hole and middle hole. |
Next, we split the class into two groups and did the Elisa test.
 |
| Elisa test setup. |
This is a test to see if there is HIV the system of a patient. This is done by detecting the antibodies for HIV in a blood sample. The basic concept of this test is that if there is HIV in the system, the body will have formed the antibodies which are meant to attach to the antigens of the HIV. We separate out this antigen, attach it to the side wall of a tube, then we add the blood. If there is antibodies in it, it will bind to the antigen, if not, then it will be washed away. To determine if the blood same left antibodies we add an animal (goat) antibody. The goat antibody is an antibody to the HIV antibody in humans. If the HIV antibody is there, the goat antibody will bind. If not, it will be washed away. This second antibody can be detected because it has an enzyme connected to it that will change color in the presence of a substrate.
Therefore, if the patient's sample is HIV positive, the human antibody will bind to the antigen, the goat antibody with the enzyme will bind to the human antibody, and the substrate will cause the solution to turn blue. If the patien'ts sample is HIV negative, there will be no antibody to attach to the antigen, and therefore not human antibody for the goat antibody with the enzyme to attach to, and everything will be washed away, leaving the solution in the tube clear of color.
For our test in particular, we had a set of 12 small tubes in a well tray. In the first 3, we added a negative sample. In the second, a positive one, and in the last the variable sample that we are trying to figure out. The positive should turn blue, the negative stay clear, and whichever the variable will look like. Our test failed and all stayed clear. The other group, besides a problem in two of the tubes, proved that their sample was HIV positive.
 |
| Other teams positive HIV test. |
*App of the day - Dr. Joseph is doing an experiment, looking into the affects of cinnamon. The oils have great antibacterial power, but a lot have adverse side effects. Some will kill red blood cells, which is a major problem. Cinnamon was found to be a good middle ground, with antibacterial power as well as the least affect on red blood cells. To use on the skin, there is no problem will cinnamon oil. Ingesting straight cinnamon oil, might be dangerous. But if you slightly increase cinnamon in your diet, it might keep bacteria in line.